Covered in the mainstream media, and then spreading virally on social media – “The FDA approves the first new type of pain medication in 25 years”!
This is, presumably, fantastic. Opioid deaths, while decreasing a small bit last year, are still at profoundly concerning levels. Hydrocodone, oxycodone, hydromorphone, morphine, and their cousins are all prescribed far too often – because, simply, clinicians have little else to offer.
The truth is much bleaker. The drug in question, Journavx (suzetrigine), is approved on the basis of a handful of pharma-conducted studies enrolling only a little over a thousand persons. These are described in some detail in the New England Journal of Medicine back in 2023, but there’s actually a surprising bit of extra information in the FDA package insert.
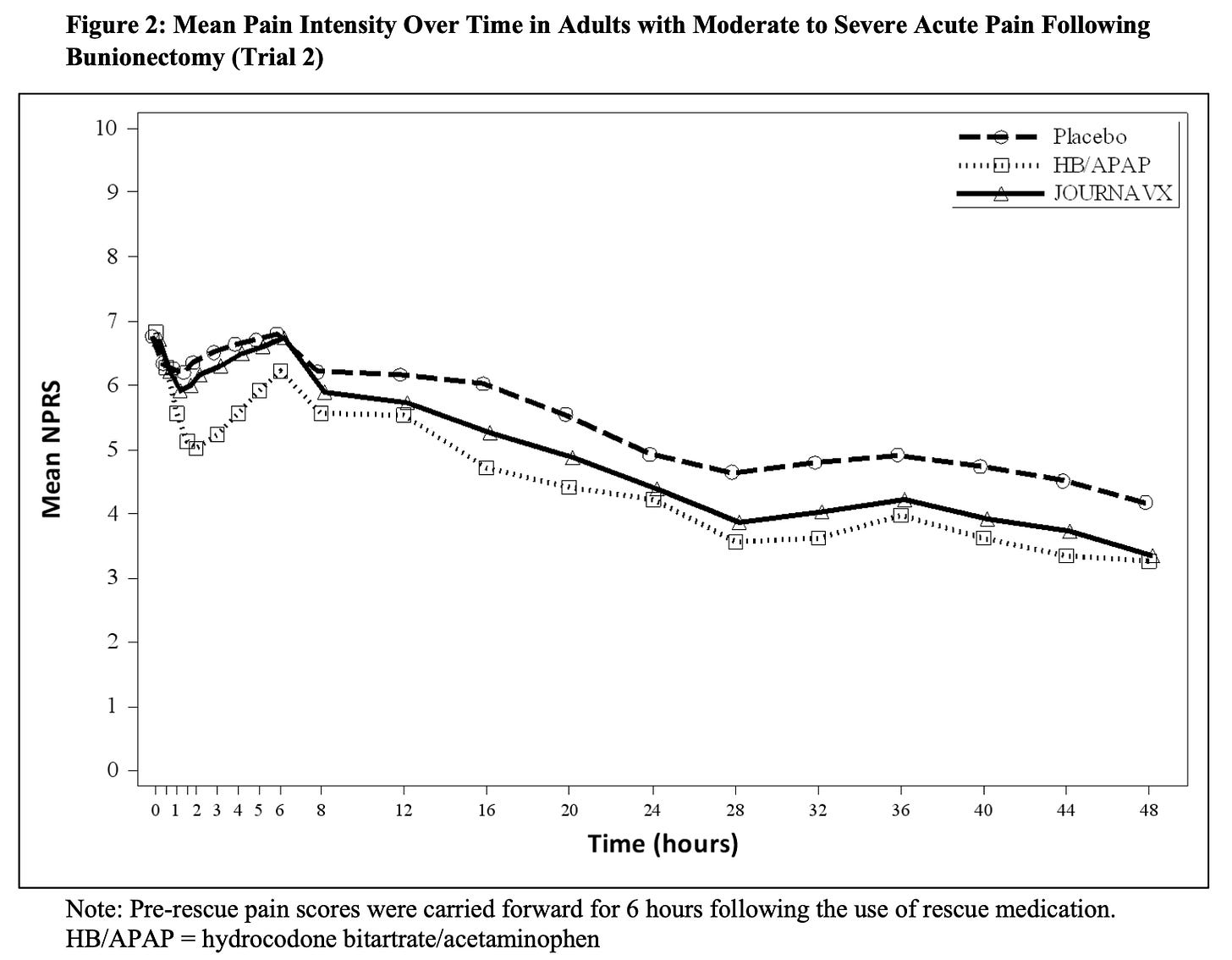
The key messages are these two figures:
On the “numerical pain rating scale”(NPRS), lower is better. From what data we are presented with, yes, Journavx is better than placebo – a tiny bit. How large does the difference need to be to be considered “minimally clinically important” on this subjective pain rating scale? This is debatable, but probably around 1.5. Differences below that indicate less-reliable expected relief from pain.
The comparison with hydrodone/acetaminophen is interesting, as well. Without trivializing any exposure to opiates, the study protocol allowed for a mere, single 5mg/325mg HB/APAP to be taken every six hours. This is a frankly insignificant dose for most adults, and doubtless comparable to non-opioid options of typical 500-1000mg acetaminophen or 400mg ibuprofen.
So, even before we worry about the biases involved with trials conducted by the pharmaceutical corporation whose livelihood depends on their successes – this is not a very effective analgesic. Then, there are quirks in the trial protocols around imputations from rescue medications, the statistical methods, and the non-representativeness of the trial populations of the rest of the world (for example, the abdominoplasty trial enrolled almost solely white females). Finally, we don’t know what effects these drugs might have taken long-term, given their unique sodium channel blockade (see: Vioxx).
A typical example of the hype outpacing the likely utility and safety of a new medicine. This may yet prove to be an option, particularly for those who cannot take NSAIDs or other alternatives due to underlying medical conditions, but there’s a long way to go before we can be confident in these findings.